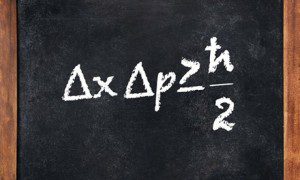At the quantum level, nature is more numinous than we realize in the material reality we experience. There is a fundamental limit to what we can know about the behaviour of quantum particles and, therefore, the smallest scales of matter and energy. It seems that the mere act of interacting with matter this small changes the way it behaves and it is impossible to observe the environment without affecting it. Measuring quantum particle waves requires that you measure both the position of a given particle and its momentum. But as Weiner Heisenberg put it:
The more precisely the position is determined, the less precisely the momentum is known in this instant, and vice versa.
–Heisenberg, uncertainty paper, 1927
This variable property of quantum particles is known as the Uncertainty Principle.
An early incarnation of the uncertainty principle appeared in Heisenberg’s 1927 paper from when he was working at Niels Bohr’s institute in Copenhagen, titled “On the Perceptual Content of Quantum Theoretical Kinematics and Mechanics”. The more familiar form of the equation came a few years later.
Among many counter-intuitive ideas, quantum theory proposed that energy was not continuous but instead came in packets (quanta) and that light could be described as both a wave and a stream of these quanta. In fleshing out this radical worldview, Heisenberg discovered a problem in the way that the basic physical properties of a particle in a quantum system could be measured.
From The Guardian:
The uncertainty principle says that we cannot measure the position (x) and the momentum (p) of a particle with absolute precision. The more accurately we know one of these values, the less accurately we know the other. Multiplying together the errors in the measurements of these values (the errors are represented by the triangle symbol in front of each property, the Greek letter “delta”) has to give a number greater than or equal to half of a constant called “h-bar”. This is equal to Planck’s constant (usually written as h) divided by 2π. Planck’s constant is an important number in quantum theory, a way to measure the granularity of the world at its smallest scales and it has the value 6.626 x 10-34 joule seconds.
Here’s a handful of great videos to explain all of this. The first one explains the structure of an atom, which is a necessary primer for the second, which explains particle-wave duality (which we discussed in the last Quantum Woo column), and then the third introduces the Uncertainty Principle:
The Structure of Atoms
Wave Function and Wave-Particle Duality
The Uncertainty Principle
A website called “How Stuff Works” I was looking at explained it in very simplistic terms:
Imagine that you’re blind and over time you’ve developed a technique for determining how far away an object is by throwing a medicine ball at it. If you throw your medicine ball at a nearby stool, the ball will return quickly, and you’ll know that it’s close. If you throw the ball at something across the street from you, it’ll take longer to return, and you’ll know that the object is far away.
The problem is that when you throw a ball — especially a heavy one like a medicine ball — at something like a stool, the ball will knock the stool across the room and may even have enough momentum to bounce back. You can say where the stool was, but not where it is now. What’s more, you could calculate the velocity of the stool after you hit it with the ball, but you have no idea what its velocity was before you hit it.
This is the problem revealed by Heisenberg’s Uncertainty Principle. To know the velocity of a quark we must measure it, and to measure it, we are forced to affect it. The same goes for observing an object’s position. Uncertainty about an object’s position and velocity makes it difficult for a physicist to determine much about the object.

But it’s even more interesting that that. If you watch the second video in the series I’ve linked, you will see that the particle that we’re trying to determine the location of, actually winks in and out of existence, can appear out of nothing, can exist simultaneously in different spaces at once, and can disappear in one space and reappear in another without existing at any time in the spaces between. Our only saving grace in throwing that medicine ball (which are the light particles we are using to observe the experiment) is that these elusive “virtual particles” can only exist within certain permitted orbits in an atom (and therefore not others), and it is these permitted spaces, and the number of electrons within them, that ultimately determine what makes one element of the periodic table different from another.
Okay, those who don’t believe in magick can leave now because I’m going to explain how I think this has a bearing in metaphysics, and so I’m sure I’ll just bore you. *wink*
Let’s consider the mind-boggling notion that particles wink in and out of existence! I find this very exciting. First of all, it tells us that matter is not as firmly determined as we originally thought. Sometimes it exists; sometimes it doesn’t. We can’t perceive this because it’s all happening on such a microscopic level, and at such a rapid rate, that in terms of interacting with the physical universe that it might as well not be going on at all. But where do the particles go when they’re not here? If energy can be neither created nor destroyed, where is it in the meantime? There are numerous ideas about this and we’ll be exploring some of them in later articles in this series. Some of them will sound really familiar to mystics and magicians, but they were conceived of by physicists.
 Also, did anyone else catch that subatomic particles can actually teleport and co-locate? Of course there are limits to this: namely the “allowable orbits” of Bohr’s atomic model. So what seems like supernatural forces actual obey a set of laws and rules; though we obviously don’t quite understand them yet. But that’s the nature of magick and most perceived “supernatural” forces, isn’t it?
Also, did anyone else catch that subatomic particles can actually teleport and co-locate? Of course there are limits to this: namely the “allowable orbits” of Bohr’s atomic model. So what seems like supernatural forces actual obey a set of laws and rules; though we obviously don’t quite understand them yet. But that’s the nature of magick and most perceived “supernatural” forces, isn’t it?
Now of course this proves nothing. The truth is that these “quanta” might not actually exist at all. They could merely be mathematical concepts created in order to interact with the environment in a way that makes sense to the creators of the idea, rather than an accurate description of the nature of how reality at the quantum level works. But this, of course, is exactly what magick is about; using a paradigm of symbology that probably isn’t actually the way things work, as a metaphor to enable us to interact with reality in productive ways. It works for physicists; why not us too?